Abstract
Background
Ibrutinib, inhibitor of Bruton's tyrosyne kinase (BTK), demonstrated exceptional activity both in untreated and relapsed/refractory CLL patients, obtaining overall response rate (ORR), progression-free survival (PFS) and overall survival (OS) far superior to chemoimmunotherapy (Byrd, 2014). Interestingly, NOTCH1 mutations (M) were significantly correlated with CD49d overexpression which identified cases with reduced lymphocytosis and inferior nodal response (Tissino, 2018). Moreover, NOTCH1 M were characterized by remarkable expression of the NF-kB pathway genes (Benedetti, 2017) and thus may confer poor response to ibrutinib.
Aims
On this background, the primary aims of our research were: i) to correlate NOTCH1 M with biological and clinical prognosticators in ibrutinib-treated patients; ii) to verify the impact of NOTCH1M both on the peripheral lymphocytes redistribution and on the nodal response calculated as percentual reduction from baseline (SPD); iii) to evaluate the impact of NOTCH1 M on ORR, PFS and OS; iiii) finally, to assess NOTCH1 M as an independent prognostic factor.
Patients and Methods
Therefore, we evaluated the efficacy of ibrutinib as single agent, in a real-life contest, on 166 patients recruited from three independent cohorts from Italy, median age 61 years (27-85), median number of previous regimens 2 (0-4; 15% previously untreated). Noteworthy, 23/60 (38%) TP53M patients were treated in first line with ibrutinib (p<0.0001). Patients received 420 mg oral ibrutinib once daily until progression or occurrence of unacceptable side effects. Median follow up on ibrutinib was 16 months. NOTCH1 M were investigated with ARMS PCR for c.75447545delCT or by Sanger sequencing of NOTCH1 exon 34 and all cases were confirmed with next generation sequencing (NGS) method.
Results
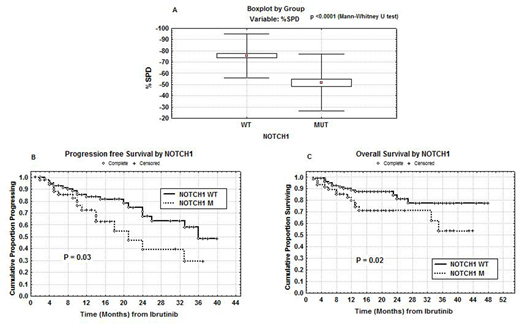
Sixty-one patients were NOTCH1 M (36.75%). NOTCH1 M were significantly correlated with trisomy 12 (p=0.00045) and with CD49d >30% (p=0.001). Absolute lymphocyte counts (ALCs) were collected before treatment and at days ranging from 30 to 90 on ibrutinib. NOTCH1 M were significantly correlated with lower median ALCs measured both before (28.9 x 106/ml vs 57.0 x 106/ml; p=0.0006) and during (28.0 x 106/ml vs 54.6 x 106/ml; p=0.0005) ibrutinib therapy. Also the median SPD, calculated at 3-6 months on ibrutinib, was significantly lower in NOTCH1 M patients (51.8% vs 75.7%; p<0.0001, Figure A). ORR was 89% (complete response (CR): 21%, partial response (PR): 31%, PR with lymphocytosis (PR-L): 37%). The estimate 1-year PFS and OS were 81% and 86%, respectively. Fifty-one patients (30.7%) discontinued ibrutinib therapy due to progression (n= 25), and adverse events (n=26). Fourteen patients in progression were treated subsequently with venetoclax. With regard to clinical outcome, PR was significantly correlated with NOTCH1 M (31/61; p=0.00002). Significant shorter PFS and OS were observed in NOTCH1 M patients (39% vs 71% and 71% vs 81% at 24 months, respectively; p=0.03 and p=0.02; Figures B and C). In multivariate analysis of PFS and OS including TP53 M, IGHV mutational status and age > or <60 years, only NOTCH1 M were confirmed as an independent prognostic factor (p=0.01 and p=0.04, respectively).
Conclusions
NOTCH1M patients were characterized by a reduced redistribution lymphocytosis and by a lower and/or slower nodal response under ibrutinib treatment. The clinical consequences were a significant higher number both of PR without lymphocytosis and relapses. Therefore, evaluation of NOTCH1 M in patients initiating ibrutinib may identify those cases that would benefit from combination therapy approaches, which are already underway in experimental protocols, using new molecules working through different pathways, such as venetoclax or obinutuzumab.
Del Principe:Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal